Welcome to the Guthold Group Research Web Site
Our research interests are at the interface of Biophysics, Molecular
Biology and nanotechnology. The major techniques we are currently using are atomic force microscopy (AFM-The nanoManipulator) for imaging and manipulating molecules, fluorescence microscopy (combined with the atomic force microscope and including TIRF illumination), single molecule imaging (AFM and fluorescence), fluorescene resonance energy transfer (FRET) and standard molecular biology techniques, such as electrophoresis, filtration techniques and protein and surface modification protocols.
We are currently working on the following projects.
-----------------------------------------------------------------------
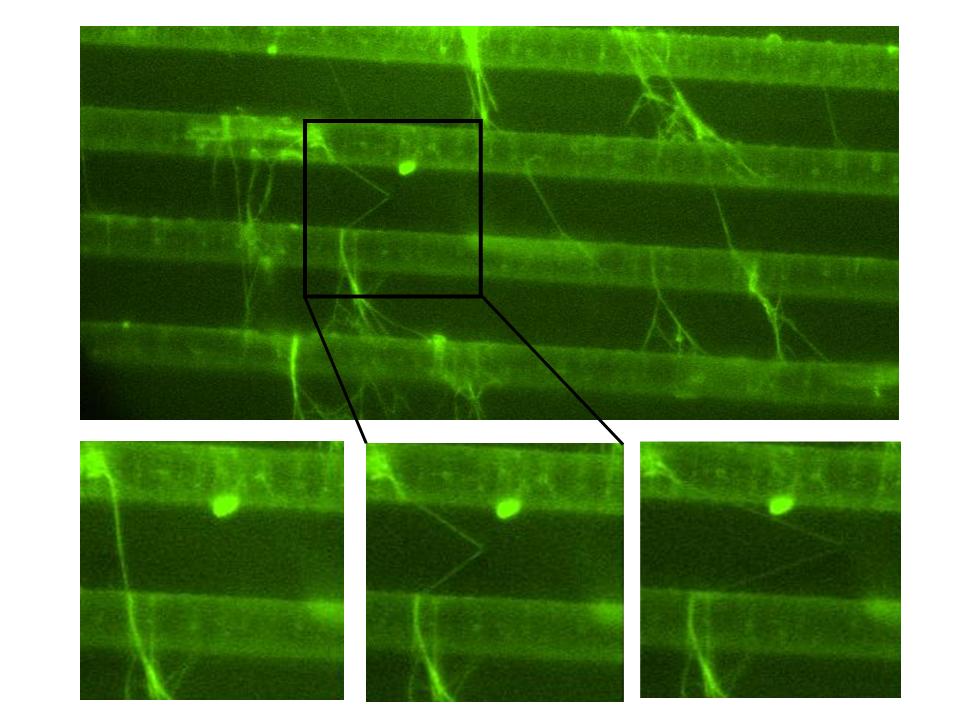 Mechanical properties of fibrin fibers and the constituents of a blood clot. Blood clots stem the flow of blood, which is essentially a mechanical task. Hence, there has been a longstanding interest, initiated by Ferry, in the mechanical properties of clots. The macroscopic properties of a clot are well known and can be related to clotting disorders. However, the underlying microscopic mechanisms, which determine the behavior of the whole clot are not understood . Clots form when soluble fibrinogen is converted to fibrin monomers that polymerize to form a branched network of fibrin fibers. The mechanical properties of such branched network depend on the network architecture and the mechanical properties of the individual fibers. These properties are poorly understood. Yet this knowledge is needed to construct and test good mechanical models of blood clots, and, thus advance our understanding of wound healing, heart attacks and strokes. We have developed an atomic force/fluorescence microscopy technique to study the mechanical properties of single fibrin fibers. We use the tip of the atomic force microscope (AFM) to stretch fibers suspended across 12 m m-wide channels; and the fluorescence microscope to image this stretching. The figure shows movie frames of a single fibrin fiber stretched to 180% length (Click to enlarge).
Mechanical properties of fibrin fibers and the constituents of a blood clot. Blood clots stem the flow of blood, which is essentially a mechanical task. Hence, there has been a longstanding interest, initiated by Ferry, in the mechanical properties of clots. The macroscopic properties of a clot are well known and can be related to clotting disorders. However, the underlying microscopic mechanisms, which determine the behavior of the whole clot are not understood . Clots form when soluble fibrinogen is converted to fibrin monomers that polymerize to form a branched network of fibrin fibers. The mechanical properties of such branched network depend on the network architecture and the mechanical properties of the individual fibers. These properties are poorly understood. Yet this knowledge is needed to construct and test good mechanical models of blood clots, and, thus advance our understanding of wound healing, heart attacks and strokes. We have developed an atomic force/fluorescence microscopy technique to study the mechanical properties of single fibrin fibers. We use the tip of the atomic force microscope (AFM) to stretch fibers suspended across 12 m m-wide channels; and the fluorescence microscope to image this stretching. The figure shows movie frames of a single fibrin fiber stretched to 180% length (Click to enlarge).
The following links show movies of fibrin fiber manipulations:
S1_Fig. 1 Breaking uncrosslinked batroxobin fiber. Uncrosslinked batroxobin fiber (The arms break at 183% and 278% strain)
S2_Fig 2 Snap-back of crosslinked thrombin. Crosslinked thrombin fiber Elastic snap-back from 80%, permanent damage at 230% strain)
S3_525%_extensibility_T_X. This fiber gets strain 525% (6.25 its length) before breaking.
S4_150% elastic 240% rupture. Crosslinked thrombin fiber (150% strain elastic, 240% permanent damage)
S5_Lolo Snap-back. Uncrosslinked thrombin (Nice, elastic snap-back)
--------------------------------------------------------------------------
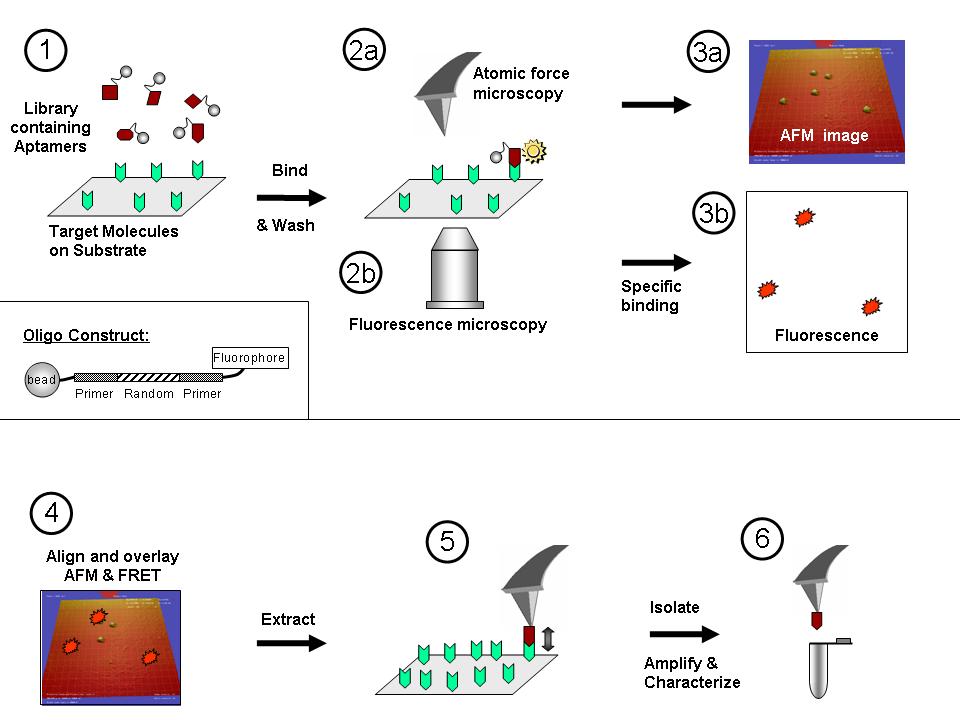 Combined atomic force-fluroescence microscopy and low copy-number PCR to select aptamers. We are developing a method, which utilizes a combined atomic force microscope (AFM)/fluorescence microscope and small copy number PCR, to affinity-select individual aptamer species in a single cycle from a small pool of random oligonucleotides (oligos). In this method, a library of small beads, each of which is functionalized with fluorescent oligos of different sequences, is created. This library of oligo-functionalized beads is flowed over immobilized target molecules on a glass cover slip. High-affinity, target-specific aptamers bind tightly to the target for prolonged periods and resist subsequent washes, resulting in a strong fluorescence signal on the substrate surface. This signal is observed from underneath the sample via fluorescence microscopy. The AFM tip, situated above the sample, is then directed to the coordinates of the fluorescence signal to collect a high-resolution image of the suface-bound bead and to subsequently extract the bead (plus attached oligo). The extracted oligo is PCR-amplified and sequenced. The figure shows the schematics of this method (click to enlarge).
Combined atomic force-fluroescence microscopy and low copy-number PCR to select aptamers. We are developing a method, which utilizes a combined atomic force microscope (AFM)/fluorescence microscope and small copy number PCR, to affinity-select individual aptamer species in a single cycle from a small pool of random oligonucleotides (oligos). In this method, a library of small beads, each of which is functionalized with fluorescent oligos of different sequences, is created. This library of oligo-functionalized beads is flowed over immobilized target molecules on a glass cover slip. High-affinity, target-specific aptamers bind tightly to the target for prolonged periods and resist subsequent washes, resulting in a strong fluorescence signal on the substrate surface. This signal is observed from underneath the sample via fluorescence microscopy. The AFM tip, situated above the sample, is then directed to the coordinates of the fluorescence signal to collect a high-resolution image of the suface-bound bead and to subsequently extract the bead (plus attached oligo). The extracted oligo is PCR-amplified and sequenced. The figure shows the schematics of this method (click to enlarge).
------------------------------------------------------------------------
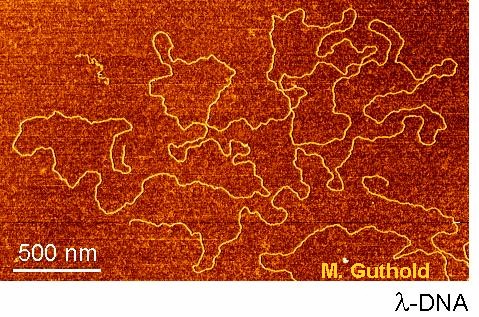 AFM and fluorescence microscopy imaging. We are always interested in using our microscopes to analyze intersting biological molecules such as DNA, DNA-protein complexes, fibrin fibers, other biological and non-biological fibers, etc.
AFM and fluorescence microscopy imaging. We are always interested in using our microscopes to analyze intersting biological molecules such as DNA, DNA-protein complexes, fibrin fibers, other biological and non-biological fibers, etc.
The images shows linearized lambda DNA on mica.
-
-
 Mechanical properties of fibrin fibers and the constituents of a blood clot. Blood clots stem the flow of blood, which is essentially a mechanical task. Hence, there has been a longstanding interest, initiated by Ferry, in the mechanical properties of clots. The macroscopic properties of a clot are well known and can be related to clotting disorders. However, the underlying microscopic mechanisms, which determine the behavior of the whole clot are not understood . Clots form when soluble fibrinogen is converted to fibrin monomers that polymerize to form a branched network of fibrin fibers. The mechanical properties of such branched network depend on the network architecture and the mechanical properties of the individual fibers. These properties are poorly understood. Yet this knowledge is needed to construct and test good mechanical models of blood clots, and, thus advance our understanding of wound healing, heart attacks and strokes. We have developed an atomic force/fluorescence microscopy technique to study the mechanical properties of single fibrin fibers. We use the tip of the atomic force microscope (AFM) to stretch fibers suspended across 12 m m-wide channels; and the fluorescence microscope to image this stretching. The figure shows movie frames of a single fibrin fiber stretched to 180% length (Click to enlarge).
Mechanical properties of fibrin fibers and the constituents of a blood clot. Blood clots stem the flow of blood, which is essentially a mechanical task. Hence, there has been a longstanding interest, initiated by Ferry, in the mechanical properties of clots. The macroscopic properties of a clot are well known and can be related to clotting disorders. However, the underlying microscopic mechanisms, which determine the behavior of the whole clot are not understood . Clots form when soluble fibrinogen is converted to fibrin monomers that polymerize to form a branched network of fibrin fibers. The mechanical properties of such branched network depend on the network architecture and the mechanical properties of the individual fibers. These properties are poorly understood. Yet this knowledge is needed to construct and test good mechanical models of blood clots, and, thus advance our understanding of wound healing, heart attacks and strokes. We have developed an atomic force/fluorescence microscopy technique to study the mechanical properties of single fibrin fibers. We use the tip of the atomic force microscope (AFM) to stretch fibers suspended across 12 m m-wide channels; and the fluorescence microscope to image this stretching. The figure shows movie frames of a single fibrin fiber stretched to 180% length (Click to enlarge). 
 AFM and fluorescence microscopy imaging. We are always interested in using our microscopes to analyze intersting biological molecules such as DNA, DNA-protein complexes, fibrin fibers, other biological and non-biological fibers, etc.
AFM and fluorescence microscopy imaging. We are always interested in using our microscopes to analyze intersting biological molecules such as DNA, DNA-protein complexes, fibrin fibers, other biological and non-biological fibers, etc.