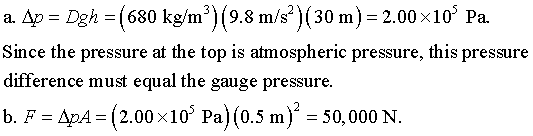C3.5

Q4.1
The four phases of matter are:
Solid: Forces hold the molecules close to each other, and the distances between atoms change only over a narrow range. Forces also hold molecules in a fixed arrangement with respect to each other. These two things mean that solids have a shaped that is rather rigid unless subjected to large forces.
Liquid: The spacing between molecules changes only over a narrow range, but the molecules can rearrange themselves easily. This means that the volume of a liquid does not change much, but its shape changes easily.
Gases: In gases, the forces between atoms and molecules are small except during collisions. The atoms are father apart. Gases have neither a well defined shape nor volume.
Plasmas: Plasmas are hot enough that the atoms themselves do not hold together, so that the plasma is a fluid of electrons and ionized atoms.
Q4.9
The atoms of gases are much farther in a gas than in a liquid or solid.
Q4.14
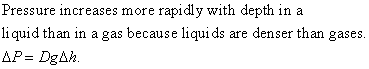
Q4.16
If g increased, then atmospheric pressure would increase, too, since atmospheric pressure is just equal to the weight per unit area of all the air above. A bigger g means a bigger weight mg. The pressure at the bottom of a swimming pool would go up for the same reason.
P4.5
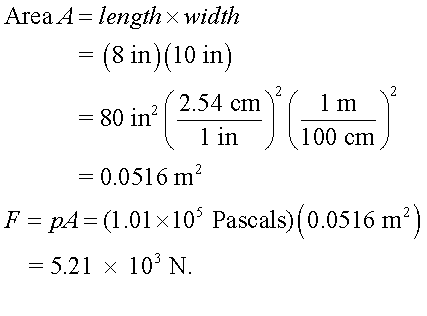
P4.10
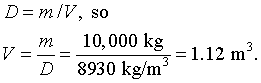
P4.18