|
Balancing Redox Equations in Acid |
| Step 1: | Separate in half reactions. |
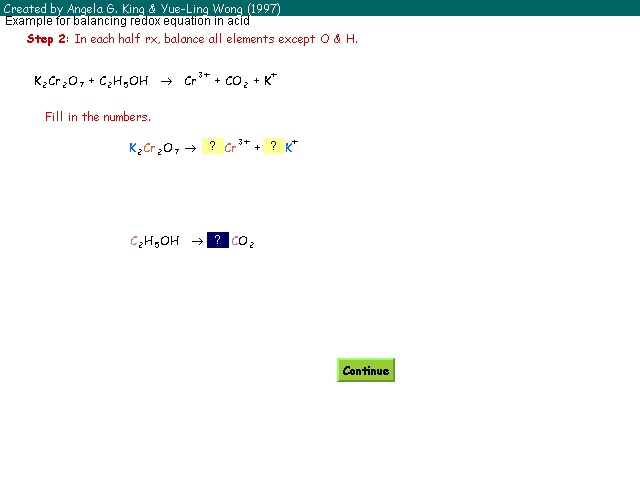
| Step 2: | In each half reaction, balance all elements except O & H. |
| Step 3: | Balance O by adding H2O. |
| Step 4: | Balance H by adding H+. |
| Step 5: | Balance charges by adding e-. |
| Step 6: | Multiply all coefficients in 1 or both half reactions by an integer to get the number of e- in the two half reactions equal. |
| Step 7: | Add the 2 half reactions together & cancel out any species that appear on both sides of net reaction. |
| Step 8: | Check that charges and atoms are balanced. |
Steps Review: An online interactive pop quiz to test your memory of the order of the steps.
Update 2020-11: The Adobe Shockwave is no longer supported but I have received requests to access this interactive tutorial. So now I have made this interactive tutorial available as an executable for Windows.
Click here to download the executable. Remember where you download the file to. After it has finished downloading, double-click on the .exe file to run it. It does not require Internet access to run.
Please email me if you need an executable for MacOS.