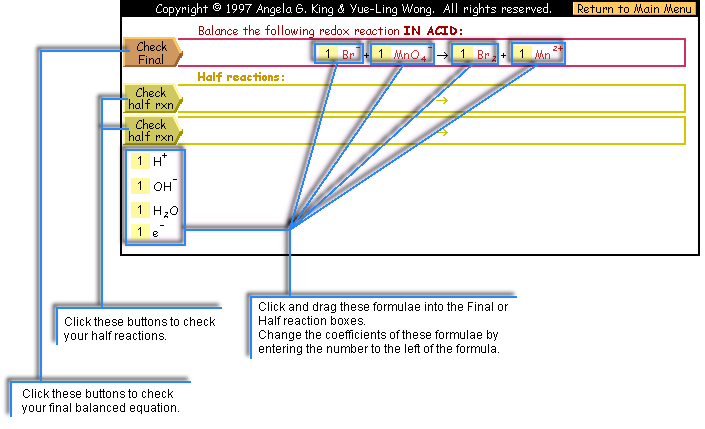
Balancing Redox Equations Interactive Practice - In Acidic and Basic Solutions
The Grading Method:
- Half Reaction: You don't have to check the half reactions. The Check Half Reaction in this online exercise only checks whether the
atoms and charges are balanced in the half reaction. It does not necessarily
mean the half reaction is correct or not. For each unbalanced atom or
charge in the half reaction, 2 points will be deducted. It helps you
to check the math of the atom counts which is the common mistakes that
lead to an incorrect final equation.
- Final Reaction:
- Coefficients and Species in the final reaction: points will be given for each of correct coefficients and chemical species in the final
reaction. The number of points for each correct answer depends on the total
number of coefficients and chemical species in the final reaction.
- H+ or OH-: if the question asks for balancing
the reaction in acid and you add OH-, 4 points will be deducted; vice versa for the H+.
- Extra H+ or OH- or H2O or e- incorrectly added to the final reaction: 4 points will be deducted for
each.
- Some intructors may teach you a different method in balancing equation
in base. You will get a different half reaction. It's OK. It will not deduct
any points from the half reaction as long as the half reactions are balanced.
You will get the same final equation no matter which method you use.

Update 2020-11: The Adobe Shockwave is no longer supported but I have received requests to access this interactive practice. So now I have made this interative practice available as an executable for Windows.
Click here to download the executable. Remember where you download the file to. After it has finished downloading, double-click on the .exe file to run it. It does not require Internet access to run.
Please email me if you need an executable for MacOS.

