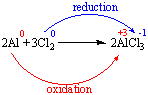
Reduction-oxidation (redox) reactions are chemical reactions in which reactants experience a change in oxidation number (which means these reactants either gain or lose electrons).
If an atom in a reactant gained electrons (its oxidation # decreased) it was reduced.
If an atom in a reactant lost electrons (its oxidation # increased) it was oxidized.
Since chemical reactions don't make or destroy electrons, oxidation and reduction must occur at the same time. As one reactant is oxidized, the electrons it loses are accepted by another reactant which is reduced.

Update 2020-11: The Adobe Shockwave is no longer supported but I have received requests to access this interactive practice. So now I have made this interative practice available as an executable for Windows.
Click here to download the executable. Remember where you download the file to. After it has finished downloading, double-click on the .exe file to run it. It does not require Internet access to run.
Please email me if you need an executable for MacOS.